Abstract
Background
Carfilzomib is an irreversible epoxyketone proteasome inhibitor which has demonstrated superior disease control and PFS for myeloma compared to bortezomib. Bendamustine is a bifunctional alkylating agent with demonstrated activity in relapsed/refractory myeloma. We hypothesized that the combination of carfilzomib and bendamustine may result in additive and synergistic effects, without excessive toxicity. In this phase I/II study, we investigate the safety and efficacy of carfilzomib, bendamustine and dexamethasone (CBD) for newly diagnosed myeloma.
Objectives
The primary objective is to determine the maximum tolerated dose (MTD) of carfilzomib in combination with bendamustine and dexamethasone. Secondary endpoints include overall response rate (ORR), duration of response (DOR), progression free survival (PFS), overall survival (OS), and assessment of stem cell collection.
Methods
Patients with newly diagnosed myeloma were enrolled. Carfilzomib was administered intravenously on days 1, 2, 8, 9, 15, 16 of a 28-day cycle. Bendamustine was given intravenously on days 1 and 2. Dexamethasone was administered orally or intravenously on days of carfilzomib and on day 22 of each cycle. The dose escalation portion of the study used an Up-and-Down dose escalation scheme (Storer's schema D), with one patient per dose level run in. Transplant eligible patients received 4 cycles of treatment and then underwent peripheral blood stem cell harvest (SCH) after mobilization with granulocyte-stimulating factor +/- plerixafor. They then received an additional 4 cycles and underwent stem cell transplant (SCT) after 8 total cycles of CBD. Transplant ineligible patients also received 8 total cycles of treatment. Both groups then received maintenance therapy with carfilzomib 36 mg/m2 on days 1, 2, 15, 16 of each 28-day cycle for up to 2 years. Adverse events were assessed according to CTCAE V4 and responses were assessed using IMWG criteria.
Results
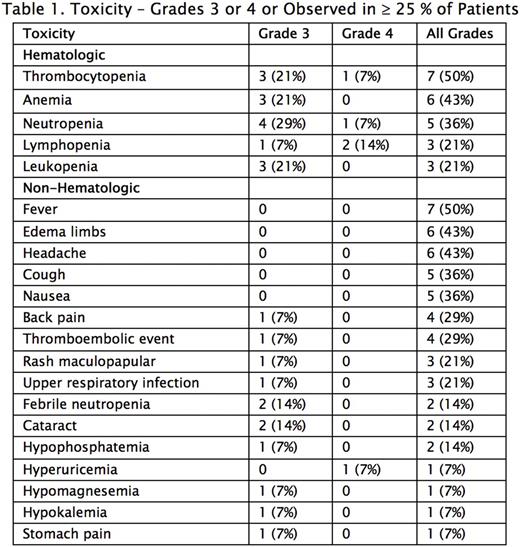
At 38 months into the study, 15 patients have been treated, amongst which 14 were evaluable for response. Median age was 65 (range: 51-74); 10/15 (67%) were male; 6 (40%) were Hispanic, 6 Caucasian, 2 (13%) Asian and 1 (7%) African American. Four patients were allocated to lower level doses and 11 patients thus far have been given the maximum planned dose - carfilzomib 56 mg/m2, bendamustine 90 mg/m2, dexamethasone 20 mg. No dose-limiting toxicities have been noted and the most common adverse events (AEs) are shown in Table 1. Hematologic AEs were most common, occurring in 9 (64%) patients, and Grade 3 or 4 events occurring in 7 (50%). Other notable AEs included Grade 1 or 2 fever (50%), Grade 1 or 2 headache (43%) and thromboembolic events (29%). There were no deaths. No congestive heart failure or arrhythmias were noted. 11 patients underwent stem cell collection, with data available for 10. Median total stem cell yield, in million CD34/kg, was 6.85 (range 5.44-13.83). Median day 1 stem cell yield was 4.43 (range 1.28-13.83). 5 patients received plerixafor. 5 patients had 2 days of collection and 1 had a third.
Response
The ORR was 14/14 (100%). 10 (71%) had a complete response (CR) / very good partial response (VGPR): 1 (7%) MRD-negative CR by flow, 1 stringent CR, 2 (14%) CR / near CR, 6 (43%) VGPR. 4 (29%) had partial response (PR). 3 of the 6 patients with VGPR and all 4 patients with PR are still on induction therapy and anticipated to have deeper responses. 3 of 4 patients with ISS stage III disease had CR with one being MRD-negative by flow. By time of SCH (after 4 cycles), 1/11 (9%) had attained CR, 7 (64%) VGPR and 3 (27%) PR. To date, 4 patients underwent SCT, and all have attained CR with 1 being MRD-negative by flow. Amongst other patients who underwent SCH, 3 were deemed ineligible for SCT, 1 declined, and 4 are eligible. Median time to best response was 3 months (range 1-11) amongst patients whose response has plateaued, and median time to CR was 4 months (range 1-11). Median follow-up was 8.8 months (range 2-37.3). To date, only 1 patient has progressed, at 18 months after start of therapy, and median PFS has not been reached. No patients have died.
Conclusions
Current preliminary evidence suggests that CBD is a potent induction regimen. CBD does not appear to impact stem cell collection, and toxicities were acceptable, although increased attention to thromboembolic events is warranted. Further evaluation of this regimen at our maximum dose level is planned.
Lentzsch: Caelum Biosciences: Other: leadership position and stock; BMS: Consultancy; Amgen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal